In situ XAFS of acid-resilient iridate pyrochlore oxygen evolution electrocatalysts under operating conditions†
Abstract
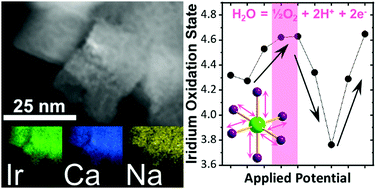
Pyrochlore iridates (Na,Ca)2−xIr2O6·H2O are acid-stable electrocatalysts that are candidates for use in electrolysers and fuel cells. Ir LIII-edge X-ray absorption fine structure spectroscopy in 1 M H2SO4 at oxygen evolution conditions suggests the involvement of the electrons from the conduction band of the metallic particles, rather than just surface iridium reacting.
- This article is part of the themed collection: Synchrotron Radiation Techniques in Catalytic Science


 Please wait while we load your content...
Please wait while we load your content...