Model for gas-hydrate equilibrium in porous media that incorporates pore-wall properties
Abstract
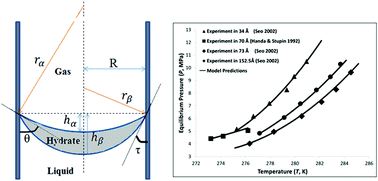
Naturally-occurring gas hydrates (in permafrost and marine sediments) have the potential to contribute as a carbon-based source to the increasing energy demand. Precise estimates of gas-hydrate global inventory, development of strategies for their exploitation, and evaluation of their environmental impact require models that accurately describe gas-hydrate stability in marine sediments. A model for gas-hydrate equilibrium in porous media, developed from fundamental thermodynamic principles, is proposed and validated against available experimental data. The derivation of the model allowed for the natural incorporation of sediment properties into equilibrium conditions, such as interfacial energies and contact angles between different phases. Model parameters were obtained from independent experiments and fundamental calculations reported in the literature. For the range of pore sizes (3.4–24.75 nm) of different materials reported in the literature, the absolute average deviations (AAD%) between the model predictions and the experimental data are between 0.04% and 2.07%. The wettability of the pore surface affects the shape of the hydrate phase inside the pore and consequently influences the equilibrium pressures of gas-hydrate formed in porous media.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...