Capacity enhancement of polylithiated functionalized boron nitride nanotubes: an efficient hydrogen storage medium†
Abstract
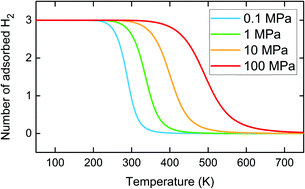
By using first principles density functional theory simulations, we report detailed geometries, electronic structures and hydrogen (H2) storage properties of boron nitride nanotubes (BNNTs) doped with selective polylithiated molecules (CLi2). We find that unsaturated bonding of Li-1s states with BNNT significantly enhances the system stability and hinders the Li–Li clustering effect, which can be detrimental for reversible H2 storage. The H2 adsorption mechanism is explained on the basis of polarization caused by the cationic Li+ of CLi2 molecules bonded with BNNT. The incident H2 molecules are adsorbed with BNNT–nCLi2 through electrostatic and van der Waals interactions. We find that with a maximum of 5.0% of CLi2 coverage on BNNT, an H2 gravimetric density of up to 4.41 wt% can be achieved with adsorption energies in the range of −0.33 eV per H2, which is suitable for ambient condition H2 storage applications.


 Please wait while we load your content...
Please wait while we load your content...