Excited-state proton transfer in protonated adrenaline revealed by cryogenic UV photodissociation spectroscopy†
Abstract
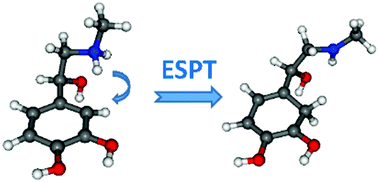
We report a comprehensive study of the structures and deactivation processes of protonated adrenaline through cryogenic UV photodissociation spectroscopy. Single UV and double-resonance UV-UV hole burning spectroscopies have been performed and compared to coupled-cluster SCS-CC2 calculations performed on the ground and first electronic states. Three conformers were assigned, two lowest energy gauche conformers along with a higher energy conformer with an extended structure which is indeed the global minimum in solution. This demonstrates the kinetic trapping of this high energy gas phase conformer during the electrospray process. At the band origin of all conformers, the main fragmentation channel is the Cα–Cβ bond cleavage, triggered by an excited state proton transfer to the catechol ring. Internal conversion leading to the water loss channel competes with the direct dissociation and tends to prevail with the increase of excess energy brought by the UV laser. Picosecond time-resolved pump–probe spectroscopy was performed to measure the excited state lifetimes of the three conformers of AdH+, which decay with the increase of excess energy in the ππ* state, from 2 ns at the band origin down to few hundreds of picoseconds 0.5 eV to the blue. Finally, about 0.8 eV above the band origin, the πσ* state is directly reached, leading to the opening of the H-loss channel.


 Please wait while we load your content...
Please wait while we load your content...