Molecular insights on NaCl crystal formation approaching PVDF membranes functionalized with graphene
Abstract
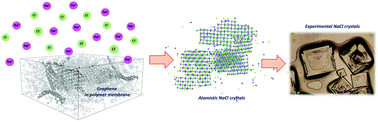
Membrane-assisted crystallization is an emerging technology where microporous hydrophobic membranes are used not as selective barriers but to promote the water vapor transfer between phases inducing supersaturation in solution. This has been successfully tested in the crystallization of ionic salts, low molecular weight organic acids and proteins. In this work, molecular dynamics simulations were used to study the crystal nucleation and growth of sodium chloride in contact with hydrophobic polymer surfaces at a supersaturated concentration of salt. A pristine polyvinylidene fluoride (PVDF) surface and PVDF containing different concentrations of graphene platelets were studied. Membrane crystallization tests were performed in parallel, in order to compare the experimental results with the computational ones. Here, with an integrated experimental–computational approach, we demonstrate that graphene-containing membranes assisted the crystal growth of NaCl, speeding up crystal nucleation in comparison with the pristine PVDF membranes. The computational results agreed with the experimental data, allowing the possibility of exploring the behavior of nanomaterials in membrane processes at a microscopic level.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...