Impact of anion shape on Li+ solvation and on transport properties for lithium–air batteries: a molecular dynamics study†
Abstract
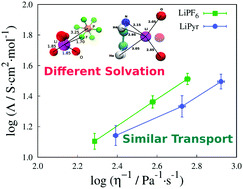
Lithium–air batteries have emerged as an interesting alternative for advanced energy storage devices. The complexity of such systems imposes great challenges. One of them resides in the selection of the lithium salt/solvent pair. Many electrolyte properties affect the operation of the batteries. Among these, the transport properties and structural features have a special place. Via molecular dynamics simulations, we have calculated solution viscosity, ionic diffusivities and conductivities, as well as structural information, for two different salts in dimethyl sulfoxide (DMSO): lithium hexafluorophosphate – LiPF6, and lithium pyrrolide – LiPyr, at different temperatures and salt molalities. We show that, despite similar ionic transport properties, Li+ solvation in the different salts is significantly different. Therefore, solutions with different solvation properties, which impact the overall battery performance, might present analogous ionic dynamics.


 Please wait while we load your content...
Please wait while we load your content...