Understanding the first half-ALD cycle of the ZnO growth on hydroxyl functionalized carbon nanotubes†
Abstract
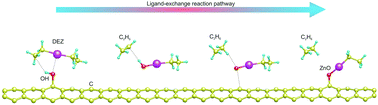
We report the adsorption of diethylzinc on hydroxyl functionalized carbon nanotubes. This study intends to understand, at the atomic level, the initial stages of ZnO formation by atomic layer deposition. Our study begins with the molecule physisorbed on the nanotube (initial state of the reaction). The final state of this reaction is when the H atom of the hydroxyl group is abstracted and migrates to one ethyl group of diethylzinc. The oxygen atom relaxes towards the nanotube and forms a strong bond with a carbon atom, while the remaining part of the molecule forms a bond with the H atom and physisorbs on top of the ZnO unit. The probability for this process to happen is very high since the energy to desorb the diethylzinc molecule is higher than the energy needed to break down the O–H bond. Non-covalent interactions and charge density distributions are plotted to confirm the break-down, formation of bonds, and repulsion during the reaction pathway.


 Please wait while we load your content...
Please wait while we load your content...