Low-energy electron scattering by cyanamide: anion spectra and dissociation pathways†
Abstract
The low-energy anion spectra of cyanamide and its rare tautomer carbodiimide were surveyed with elastic electron scattering calculations. Our assignments differ qualitatively and quantitatively from a previous theoretical report. We support that both tautomers present two π* and two 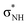 shape resonances, while cyanamide should also display a dipole bound state and a
shape resonances, while cyanamide should also display a dipole bound state and a 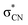 shape resonance. Available dissociative electron attachment measurements have shown several structures for dehydrogenation below 4 eV, but no sharp peaks related to vibrational Feshbach resonances. The absence of these resonances is explained by the lack of a potential barrier for tunneling of the hydrogen atom, despite the coupling between dipole bound and
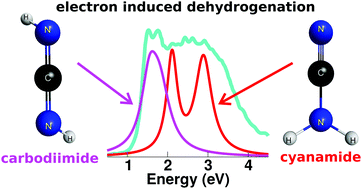
shape resonance. Available dissociative electron attachment measurements have shown several structures for dehydrogenation below 4 eV, but no sharp peaks related to vibrational Feshbach resonances. The absence of these resonances is explained by the lack of a potential barrier for tunneling of the hydrogen atom, despite the coupling between dipole bound and 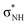 states. We found that the π* resonances initiate the dynamics that lead to hydrogen loss at 1.5, 2.5 and 3 eV. The later two structures arise from the anion states of cyanamide, while carbodiimide should account for the lower-lying one. The rarity of the second tautomer would be offset by its larger dissociative electron attachment cross section, enough to leave a distinct signature in the measured ion yield spectra. Low-energy electrons should thus decompose carbodiimide much more efficiently than cyanamide.
states. We found that the π* resonances initiate the dynamics that lead to hydrogen loss at 1.5, 2.5 and 3 eV. The later two structures arise from the anion states of cyanamide, while carbodiimide should account for the lower-lying one. The rarity of the second tautomer would be offset by its larger dissociative electron attachment cross section, enough to leave a distinct signature in the measured ion yield spectra. Low-energy electrons should thus decompose carbodiimide much more efficiently than cyanamide.


 Please wait while we load your content...
Please wait while we load your content...