A quantum chemical study of substituent effects on CN bonds in aryl isocyanide molecules adsorbed on the Pt surface†
Abstract
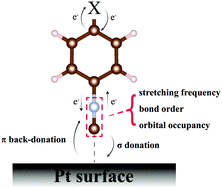
A periodicity implemented scheme of natural bond orbital (NBO) theory and normal mode analysis has been employed to investigate the tendency of the chemical bond strength of aryl isocyanide molecules with different para-substituted groups adsorbed on the Pt(111) surface. The NC bond order shows a clear correspondence with the NC stretching frequency; both of them exhibit a “volcano-like” profile as a function of the Hammett constant of the para-substituted groups for isolated molecules. When a molecule is adsorbed on the Pt(111) surface, the NC stretching frequency variations are determined by the resultant effect of σ donation and π back-donation between the molecule and the surface. The present comprehensive and systematic computations clarify the electron donating and withdrawing effects of the substituted groups on the interaction between the aryl isocyanide molecule and the transition metal substrate.


 Please wait while we load your content...
Please wait while we load your content...