Good stability and high thermoelectric performance of Fe doped Cu1.80S
Abstract
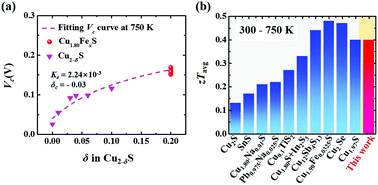
Copper sulfides have attracted great attention recently in the thermoelectric community due to the liquid-like behavior of Cu ions. Among the numerous copper sulfides, digenite Cu1.80S has a poorer thermoelectric performance but better stability than the state-of-the-art binary copper sulfide Cu1.97S. In this study, good stability and high thermoelectric performance were simultaneously obtained in Fe-doped Cu1.80S. Because Fe ions will not form a concentration gradient under an external field to change the critical voltage, Fe-doped Cu1.80S samples inherit the good stability of the pristine Cu1.80S. The critical voltage for Cu1.80Fe0.064S is 0.16 V at 750 K, which has been the largest value reported so far. Likewise, the Fe dopants can significantly improve the thermoelectric performance by suppressing the too high electrical conductivity of Cu1.80S. The peak dimensionless figure of merit (zT) for Cu1.80Fe0.064S is around 0.8 at 750 K, about four times that of Cu1.80S. The average zT for Cu1.80Fe0.064S is 0.40 in 300–750 K, which is amongst the highest values in reported thermoelectric sulfides. Combining the good stability and high thermoelectric performance, the present Cu1.80Fe0.064S has great potential to be used in the application of waste heat harvesting in the middle temperature range.


 Please wait while we load your content...
Please wait while we load your content...