The eternal battle to combat global warming: (thio)urea as a CO2 wet scrubbing agent†
Abstract
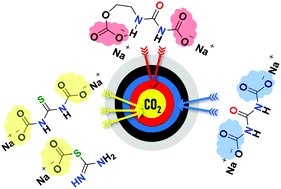
(Thio)Urea scaffolds are best known for their importance as intermediates in organic synthesis. In this work, a mechanistic study of the reaction between urea (U), (2-hydroxyethyl)urea (U-EtOH) and thiourea (tU)/NaH in DMSO with CO2 was carried out. While both U/tU reacted with CO2via a 1 : 2 mechanism through the formation of the keto (thio)carbamide–carboxylate adducts (k-U/tU-CO2− Na+), U-EtOH gave mixed CO2-adducts composed of organic carbonate and carbamide–carboxylate moieties (Na+-CO2-U-Et-OCO2− Na+). Moreover, we recorded for the first time, a new type of bond, namely sodium carbamimidothiocarbonate (e-tU-SCO2− Na+), upon bubbling CO2 in the DMSO solution of tU due to the persistence of the enol form (e-tU) and the better nucleophilicity of sulfur over nitrogen focal points. The reaction mechanisms were proven by 1D and 2D nuclear magnetic resonance (NMR) and ex situ attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopies. The stability of these bonds was studied following the changes in 1H-NMR as a function of temperature, which indicated the reversibility of these reactions. Furthermore, the proposed mechanisms were explored theoretically via density functional theory (DFT) calculations by analyzing the energetics of the anticipated products.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...