Yields of primary species in the low-linear energy transfer radiolysis of water in the temperature range of 25–700 °C
Abstract
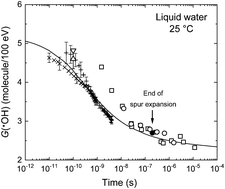
Monte Carlo track chemistry simulations were used to calculate the time-dependent yields (G values) for the radical (eaq−, H˙, ˙OH) and molecular (H2, H2O2) “primary species” formed in the low-linear energy transfer (LET) radiolysis of deaerated, pure water (H2O) in the range of ∼1 ps to 1 ms between 25 and 700 °C, at 25 MPa pressure. Beyond the critical point, we used in the calculations the new supercritical water (SCW) radiolysis database of Liu et al., in particular their reported reaction rate constants. A striking conclusion of these simulations is the sharp increase in G(˙OH) and G(H2), and the corresponding decrease in G(H˙), which are observed above 200 °C, due to the oxidation of water by the H˙ atom (H˙ + H2O → ˙OH + H2) in the homogeneous chemical stage of radiolysis. These results may have important implications for proposed Generation-IV SCW-cooled reactors for the control and management of water chemistry and for the maintenance of the structural integrity of materials.


 Please wait while we load your content...
Please wait while we load your content...