Intercalation, decomposition, entrapment – a new route to graphene nanobubbles†
Abstract
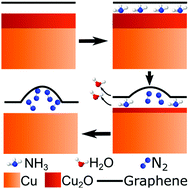
Graphene nanobubbles (GNBs) have become the subject of recent research due to their novel physical properties. However, present methods to create them involve either extreme conditions or complex sample fabrication. We present a novel approach which relies on the intercalation of small molecules (NH3), their surface-mediated decomposition and the formation of larger molecules (N2) which are then entrapped beneath the graphene in bubbles. Our hypothesised reaction mechanism requires the copper substrate, on which our graphene is grown via chemical vapour deposition (CVD), to be oxidised before the reaction can occur. This was confirmed through X-ray photoelectron spectroscopy (XPS) data of both oxidised and reduced Cu substrate samples. The GNBs have been analysed through atomic force microscopy (AFM, after NH3 treatment) and XPS, which reveals the formation of five distinct N 1s peaks, attributed to N2 entrapment, N doping species and atomic nitrogen bonded with the Cu within the substrate. This method is simple, occurs at low temperatures (520 K) and integrates very easily with conventional CVD graphene growth, so presents an opportunity to open up this field of research further.


 Please wait while we load your content...
Please wait while we load your content...