Understanding the vapochromic response of mixed copper(i) iodide/silver(i) Iodide nanoparticles toward dimethyl sulfide†
Abstract
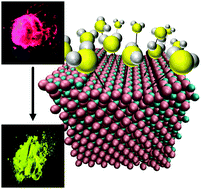
We report on the vapochromic behavior of a series of homo- and heterometallic copper(I) iodide/silver(I) iodide nanoparticles when exposed to dimethyl sulfide (DMS) vapor. These systems show remarkable colorimetric sensing behavior via emission color upon DMS exposure, shifting from pink to green emission. Kinetics measurements of CuI/AgI nanoparticle reactions with DMS show a significant rate increase with increasing Ag(I) content. However, luminescence spectroscopy and X-ray diffraction of the post-exposure samples with varying Ag(I) content reveal that the luminophore is identical in all cases and contains no Ag(I) ions. To rationalize the experimental observations and determine the vapochromic response mechanism, molecular dynamic calculations were performed on model (111) cation-terminated surfaces of copper iodide crystals doped with variable amounts of silver. Computational studies indicate that heterometallic Cu/Ag systems have a stronger binding affinity towards DMS vapor molecules than homometallic CuI and that embedding of the DMS molecules into the surface is the primary intermediate by which the vapochromic response occurs.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...