Water does not catalyze the reaction of OH radicals with ethanol
Abstract
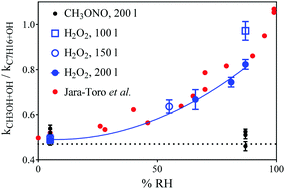
Recent experiments suggested that water catalyzes the reaction of OH radicals with alcohols, while another work showed the opposite result. Here, we resolve this disagreement and show that heterogeneous oxidation systematically biased the work showing the catalytic effect and corroborate that water does not catalyze the reaction of OH with alcohols.


 Please wait while we load your content...
Please wait while we load your content...