A detailed look at the bonding interactions in the microsolvation of monoatomic cations
Abstract
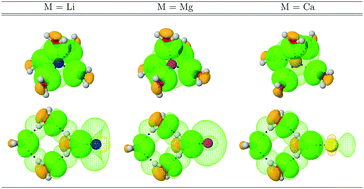
Global and local descriptors of the properties of intermolecular bonding, formally derived from independent methodologies (QTAIM, NCI, NBO, density differences) afford a highly complex picture of the bonding interactions responsible for microsolvation of monoatomic cations. In all cases, the dominant factor dictating geometries and interaction strengths is the electrophilic power of the metal cation. The formal charge disrupts the hydrogen bonding network otherwise present in pristine water clusters, making the hydrogen bonds considerably stronger, even inducing some degree of covalency. All M⋯O interactions are highly ionic, with strengths than in some cases approach that of the reference LiCl bond. Accumulation of electron density in the region connecting M⋯O is observed, thus, ionic bonding in the microsolvation of monoatomic cations is not as simple as an electrostatic interaction between opposing charges.


 Please wait while we load your content...
Please wait while we load your content...