Substitution pattern controlled aggregation-induced emission in donor–acceptor–donor dyes with one and two propeller-like triphenylamine donors†
Abstract
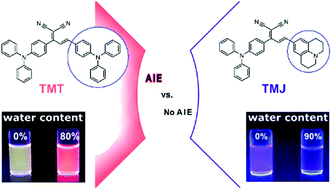
We present a comparative study of the spectroscopic properties of the donor–acceptor–donor substituted dyes triphenylamine-allylidenemalononitrile-julolidine (TMJ) and triphenylamine-allylidenemalononitrile-triphenylamine (TMT), bearing one and two propeller-like triphenylamine donor moieties, in solvents of varying polarity and viscosity and in the aggregated and solid state. Our results reveal control of the aggregation-induced spectroscopic changes and the packing motifs of the dye molecules in the solid state by the chemical nature and structure of the second nitrogen-containing donor, i.e., a planar and a rigid julolidine or a twisted triphenyl group. Assuming that the TMT and TMJ aggregates show a comparable arrangement of the molecules to the respective crystals, these different molecular interactions in the solid state are responsible for aggregation induced emission (AIE) in the case of TMT and its absence for TMJ. Moreover, a versatile strategy for the fluorescence enhancement of only weakly emissive AIE dyes is shown, turning these dyes into bright nanoscale fluorescent reporters by using them as stains for preformed polymer particles.


 Please wait while we load your content...
Please wait while we load your content...