Counting cations involved in cationic clusters of hydroxy-functionalized ionic liquids by means of infrared and solid-state NMR spectroscopy†
Abstract
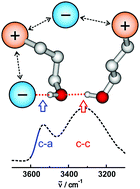
In hydroxy-functionalized ionic liquids, two types of hydrogen bonding coexist: the conventional H-bonds between cation and anion (c–a) and those between cation and cation (c–c), although the interaction between like-charged ions is supposed to be much weaker due to the repulsive Coulomb forces. Counting the cations involved in either (c–a) or (c–c) clusters is a challenge. For that purpose, we recently performed neutron diffraction (ND) measurements and molecular dynamics (MD) simulations at and above room temperature accompanied by NMR solid-state experiments in the glassy state of the ILs. In principle, these methods are suitable for determining the populations of (c–a) and (c–c) cluster species. For different reasons we could only address single temperatures and/or small temperature intervals above 300 K. The by far largest temperature range with reasonable efforts is accessible by simple infrared (IR) spectroscopy. However, counting (c–a) or (c–c) hydrogen bonds is a difficult task due to the different transition dipole moments resulting in varying intensities and broad vibrational bands. Here we present a method for deriving the number of cations involved in (c–a) ion pairs from IR spectra in the OH stretch region. This procedure provides access to the equilibria of (c–a) and (c–c) hydrogen bonds as a function of temperature allowing derivation of the transition enthalpy.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...