Theoretical study of the mechanism behind the site- and enantio-selectivity of C–H functionalization catalysed by chiral dirhodium catalyst†
Abstract
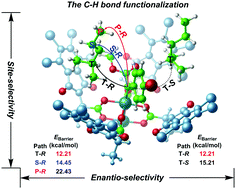
The C–H functionalization is very important for the synthesis of pharmaceuticals and complex natural products. Rhodium carbenoids, obtained when a dirhodium(II) catalyst containing a crown formed by chiral ligands reacts with diazo compounds with both an electron donating group and an electron withdrawing group, play an important part in controlling site- and enantio-selectivity for functionalization of non-activated C–H bonds. It has earlier been demonstrated that the tertiary C–H bond is more favored to be functionalized inside the crown of the dirhodium catalyst with S-configuration ligands compared with the secondary and primary C–H bonds although the latter possess weaker steric effects. We argue that the higher site- and enantio-selectivity for some types of C–H bond functionalization can be related to intermolecular hydrogen bonding, steric hindrance, and weak interactions when the dirhodium catalyst is interacting with the chiral ligands.


 Please wait while we load your content...
Please wait while we load your content...