Boosting the activity of transition metal carbides towards methane activation by nanostructuring†
Abstract
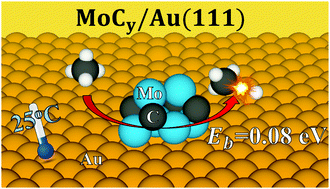
The interaction of methane with pristine surfaces of bulk MoC and Mo2C is known to be weak. In contrast, a series of X-ray photoelectron spectroscopy (XPS) experiments, combined with thermal desorption mass spectroscopy (TDS), for MoCy (y = 0.5–1.3) nanoparticles supported on Au(111)—which is completely inert towards CH4—show that these systems adsorb and dissociate CH4 at room temperature and low CH4 partial pressure. This industrially-relevant finding has been further investigated with accurate density functional theory (DFT) based calculations on a variety of MoCy supported model systems. The DFT calculations reveal that the MoCy/Au(111) systems can feature low C–H bond scission energy barriers, smaller than the CH4 adsorption energy. Our theoretical results for bulk surfaces of Mo2C and MoC show that a simple Brønsted–Evans–Polanyi (BEP) relationship holds for C–H bond scission on these systems. However, this is not the case for methane activation on the MoCy nanoparticles as a consequence of their unique electronic and chemical properties. The discovery that supported molybdenum carbide nanoparticles are able to activate methane at room temperature paves the road towards the design of a new family of active carbide catalysts for methane activation and valorisation, with important implications in climate change mitigation and carbon cycle closure.


 Please wait while we load your content...
Please wait while we load your content...