Liquid-phase decomposition mechanism for bis(triaminoguanidinium) azotetrazolate (TAGzT)†
Abstract
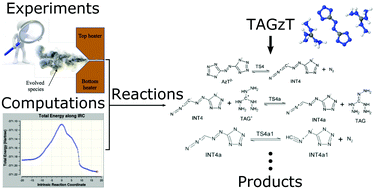
This work provides new insights for the liquid-phase decomposition of bis(triaminoguanidinium) azotetrazolate (TAGzT). The liquid-phase decomposition process was investigated using a combined experimental and computational approach. Sub-milligram samples of TAGzT were heated at rates of about 2000 K s−1 to a set temperature (230 to 260 °C) where liquid-phase decomposition occurred under isothermal conditions. Fourier transform infrared (FTIR) spectroscopy and time-of-flight mass spectrometry (ToFMS) were used to acquire transmittance spectra and mass spectra of the evolved gas-phase species from the rapid thermolysis, respectively. FTIR spectroscopy was also used to acquire the transmittance spectra of the condensate and residue formed from the decomposition. N2, NH3, HCN, N2H4, triaminoguanidine and 3-azido-1,2,4-triazol-4-ide anion were identified as products of liquid-phase decomposition. Quantum chemical calculations were used for confirming the identity of the species observed in experiments and for identifying elementary chemical reactions that formed these species. Based on the calculated free energy barriers of these elementary reactions, important reaction pathways were identified for the formation of each of the product species.


 Please wait while we load your content...
Please wait while we load your content...