Giant optical absorption and ferroelectric polarization of BiCoO2S perovskite oxysulfide by first principles prediction†
Abstract
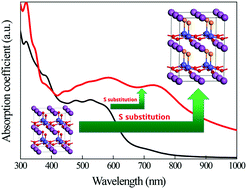
Obtaining an ideal ferroelectric photovoltaic (FE-PV) material with a narrow bandgap and a large ferroelectric polarization value can enable us to achieve great practical FE-PV performance. By the introduction of sulfur into the tetragonal BiCoO3 perovskite with a C-type antiferromagnetic ordering, it is found that the bandgap of BiCoO2S decreases significantly (about 1.2 eV) while maintaining a large polarization value (about 1.86 C m−2) that is similar to the value of 1.793 C m−2 of BiCoO3. Most noteworthy is that the optical absorption of BiCoO2S is remarkably higher than those of BiCoO3 and other FE-PV materials. The decrease of the BiCoO2S bandgap originates from the movement of Co 3d states to a low-energy position due to the reduction of the Co ionicity when the less electronegative sulfur is introduced into BiCoO3 to substitute oxygen. The narrow bandgap and the high optical absorption of the BiCoO2S films grown on different substrates are favorable for FE-PV applications. In addition, the bandgap of BiCoO2S can be modulated by the doping amount of sulfur, which can help us fabricate multilayer FE-PV devices based on different bandgaps from different layers.


 Please wait while we load your content...
Please wait while we load your content...