Formation of resonances and anionic fragments upon electron attachment to benzaldehyde†
Abstract
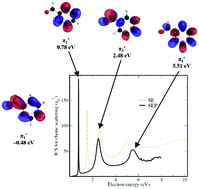
Benzaldehyde is a simple aromatic aldehyde and has a wide range of applications in the food, pharmaceutical, and chemical industries. The positive electron affinity of this compound suggests that low-energy electrons can be easily trapped by neutral benzaldehyde. In the present study, we investigated the formation of negative ions following electron attachment to benzaldehyde in the gas-phase. Calculations on elastic electron scattering from benzaldehyde indicate a π* valence bound state of the anion at −0.48 eV and three π* shape resonances (0.78, 2.48 and 5.51 eV). The excited state spectrum of the neutral benzaldehyde is also reported to complement our findings. Using mass spectrometry, we observed the formation of the intact anionic benzaldehyde at ∼0 eV. We ascribe the detection of the benzaldehyde anion to stabilization of the π* valence bound state upon dissociative electron attachment to a benzaldehyde dimer. In addition, we report the cross sections for nine fragment anions formed through electron attachment to benzaldehyde. Investigations carried out with partially deuterated benzaldehyde show that the hydrogen loss is site-selective with respect to the incident electron energy. In addition, we propose several dissociation pathways, backed up by quantum chemical calculations on their thermodynamic thresholds. The threshold calculations also support that the resonances formed at higher energies lead to fragment anions observable by mass spectrometry, whereas the resonances at low electron energies decay only by electron autodetachment.


 Please wait while we load your content...
Please wait while we load your content...