CO2 as an auto-catalyst for the oxidation of CO by a Criegee intermediate (CH2OO)†
Abstract
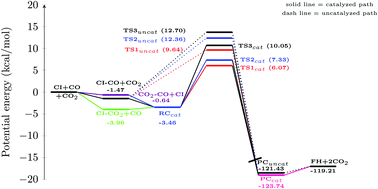
The present work investigates the effect of CO2 on the CH2OO + CO reaction, employing the CCSD(T)/CBS//M06-2X/aug-cc-pVTZ level of theory. Our calculations reveal that, in the presence of CO2, the reaction barrier of the title reaction can be reduced by up to ∼5.0 kcal mol−1. In addition, it has been found that in the presence of a catalyst, three different paths become available by which the reaction can proceed. Besides, the estimated rate constant values reveal that the bimolecular rate constant for the catalyzed path can be ∼20.0 times higher than that for the uncatalyzed channel.


 Please wait while we load your content...
Please wait while we load your content...