Structural and electronic properties of CuII, CoII, and NiII-containing chelate-based ionic liquids†
Abstract
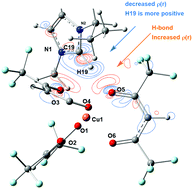
Potential applications of chelate-based ionic liquids depend on the structural and electronic properties of this class of liquid materials. Due to the large size of chelated metal anions, the high number of potential interaction sites could lead to complex intermolecular interactions, but metal-based anions have many degrees of freedom, and they have great potential in the structural modification of the physical properties of ionic liquids. To explore the influence of varying the metal center of anions and the length of carbon chains of cationic species, four single crystal structures of chelate-based ionic liquids, ([C10mim][M(F6-acac)3], M = Cu, Co, and Ni) and [C6mim][Cu(F6-acac)3] were obtained. Taking these as the initial configurations, theoretical efforts were made to understand the structural and electronic properties. The hydrogen bonding energies of the primary hydrogen bonding interaction, in the range of 17.7–20.9 kJ mol−1, follow the order of [C10mim][Ni(F6-acac)3] < [C10mim][Co(F6-acac)3] <[C10mim][Cu(F6-acac)3], and [Cnmim][Cu(F6-acac)3] (n ≠ 10) < [C10mim][Cu(F6-acac)3], while the experimental viscosities exhibit an opposite trend. Furthermore, by NBO analysis, the more negative charge on the oxygen atoms of anions shows the stronger hydrogen bonding of imidazolium with C2H. The reliability of the theoretical method was supported by the comparison between the simulated and experimental infrared and UV/vis spectra. This work is useful in increasing the understanding of the structure–property relationship of chelate-based ionic liquids and furthering the rational design of novel ionic liquids.


 Please wait while we load your content...
Please wait while we load your content...