Insights into directional movement in molecular machines from free-energy calculations†
Abstract
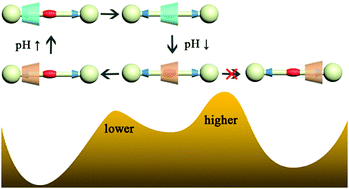
A rotaxane composed of a symmetrical axle containing three binding stations and a cone-like macrocycle containing two secondary amines has been investigated at the atomic level. At high pH, the macrocycle binds to the intermediate di(quaternary ammonium) site, while at low pH, the protonated macrocycle selectively moves along the axle to one of the two symmetrical phenyl triazole binding sites facing its upper rim, but does not shuttle backward. The determined free-energy profile characterizing the translocation of the macrocycle indicates that the selected binding site is energetically more favorable than the one facing the lower rim of the macrocycle and the free-energy barrier against translocation to the former site is lower than to the latter one, rationalizing the directional movement. This selectivity mainly stems from the asymmetry of the macrocycle shape. The strong electrostatic repulsion between the ring and the axle is found to constitute the driving force for the shuttling of the ring and also the resistance for its reverse motion. Moreover, the effect of the solvent on the shuttling has been examined, suggesting that increasing the solvent polarity may weaken the directional preference of shuttling, due to the shielding effect of polar solvents on electrostatic interactions. Our study provides a theoretical framework for tuning the selectivity of directional movement in molecular machines.


 Please wait while we load your content...
Please wait while we load your content...