On the development of a gold-standard potential energy surface for the OH− + CH3I reaction†
Abstract
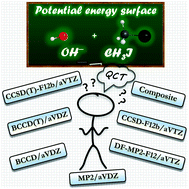
We report a story where CCSD(T) breaks down at certain geometries of the potential energy surface (PES) of the OH− + CH3I reaction. To solve this problem, we combine CCSD-F12b and Brueckner-type BCCD(T) methods to develop a full-dimensional analytical PES providing method- and basis-converged statistically-accurate SN2 and proton-transfer cross sections.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...