Mechanistic study of cellobiose conversion to 5-hydroxymethylfurfural catalyzed by a Brønsted acid with counteranions in an aqueous solution†
Abstract
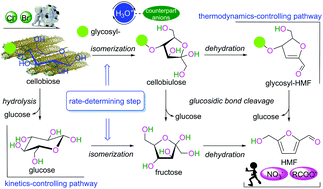
The fundamental understanding of the cooperativity of a Brønsted acid together with its anion for cellulose conversion in an aqueous solution is limited at present, in which cellobiose has usually been regarded as a bridge that connects monosaccharides and cellulose. The mechanism of β-cellobiose conversion to 5-hydroxymethylfurfural (HMF) catalyzed by a Brønsted acid (H3O+) accompanied by counteranions in an aqueous solution has been studied using quantum chemical calculations at the M06-2X/6-311++G(d,p) level under a polarized continuum model (PCM-SMD). For the formation of the first HMF from cellobiose, there are three reaction pathways, i.e., through cellobiulose and glycosyl–HMF (C/H), through cellobiulose and fructose (C/F/H), and through glucose (C/G/H). For these three reaction pathways, the rate-determining steps are associated with the intramolecular [1,2]-H shift in the aldose–ketose tautomerization. C/H is the thermodynamically predominant pathway, while C/G/H is the kinetically dominant pathway. From cellobiose, the origin of the first HMF results kinetically from a small proportion of both C/H and C/F/H and from a large proportion of C/G/H. For the role of the counteranion in the catalytic activity of H3O+, the halide anions (Cl− and Br−) act as promoters, whereas both NO3− anions and carboxylate-containing anions behave as inhibitors. The roles of these anions in β-cellobiose conversion to HMF can be correlated with their electrostatic potential and atomic number, which may cause a decrease in the relative enthalpy energy and the value of entropy on interacting with the cation moiety. These insights may advance the novel design of sustainable conversion systems for cellulose conversion into HMF.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...