Carbon dioxide, bicarbonate and carbonate ions in aqueous solutions under deep Earth conditions†
Abstract
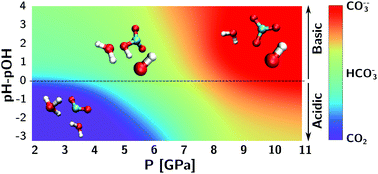
We investigate the effect of pressure, temperature and acidity on the composition of water-rich carbon-bearing fluids under thermodynamic conditions that correspond to the Earth's deep crust and upper mantle. Our first-principles molecular dynamics simulations provide mechanistic insight into the hydration shell of carbon dioxide, bicarbonate and carbonate ions, and into the pathways of the acid/base reactions that convert these carbon species into one another in aqueous solutions. At temperatures of 1000 K and higher, our simulations can sample the chemical equilibrium of these acid/base reactions, thus allowing us to estimate the chemical composition of diluted carbon dioxide and (bi)carbonate ions as a function of acidity and thermodynamic conditions. We find that, especially at the highest temperature, the acidity of the solution is essential to determine the stability domain of CO2vs. HCO3−vs. CO32−.
- This article is part of the themed collection: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...