Intramolecular hydrogen tunneling in 2-chloromalonaldehyde trapped in solid para-hydrogen†
Abstract
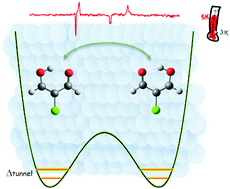
The internal dynamics of a 2-chloromalonaldehyde (2-ClMA) molecule, possessing a strong internal hydrogen bond (IHB), was examined by means of matrix isolation spectroscopy in a soft host: para-hydrogen (pH2). 2-ClMA is a chlorinated derivative of malonaldehyde (MA), a model molecule in hydrogen transfer studies, better suited to low temperature experiments than its parent molecule. The infrared absorption spectra of 2-ClMA isolated in pH2 exhibit temperature dependent structures which are explained as transitions occurring from split vibrational levels induced by hydrogen tunneling. The doublet components associated with higher and lower energy levels are changing reversibly with the increase/decrease of the matrix temperature. The ground state splitting is measured to be 7.9 ± 0.1 cm−1. The presence of oH2 impurities in the pH2 matrix close to the neighborhood of the 2-ClMA molecule is found to quench the H tunneling. The data provide a powerful insight into the dynamical picture of intramolecular hydrogen tunneling in a molecule embedded in a very weakly perturbing environment.

 Please wait while we load your content...
Please wait while we load your content...