Reaction mechanism of the Me3AuPMe3–H2 plasma-enhanced ALD process†
Abstract
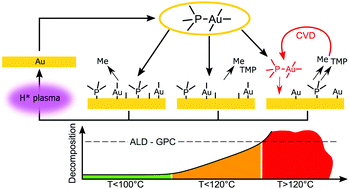
The reaction mechanism of the recently reported Me3AuPMe3–H2 plasma gold ALD process was investigated using in situ characterization techniques in a pump-type ALD system. In situ RAIRS and in vacuo XPS measurements confirm that the CH3 and PMe3 ligands remain on the gold surface after chemisorption of the precursor, causing self-limiting adsorption. Remaining surface groups are removed by the H2 plasma in the form of CH4 and likely as PHxMey groups, allowing chemisorption of new precursor molecules during the next exposure. The decomposition behaviour of the Me3AuPMe3 precursor on a Au surface is also presented and linked to the stability of the precursor ligands that govern the self-limiting growth during ALD. Desorption of the CH3 ligands occurs at all substrate temperatures during evacuation to high vacuum, occurring faster at higher temperatures. The PMe3 ligand is found to be less stable on a gold surface at higher substrate temperatures and is accompanied by an increase in precusor decomposition on a gold surface, indicating that the temperature dependent stability of the precursor ligands is an important factor to ensure self-limiting precursor adsorption during ALD. Remarkably, precursor decomposition does not occur on a SiO2 surface, in situ transmission absorption infrared experiments indicate that nucleation on a SiO2 surface occurs on Si–OH groups. Finally, we comment on the use of different co-reactants during PE-ALD of Au and we report on different PE-ALD growth with the reported O2 plasma and H2O process in pump-type versus flow-type ALD systems.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...