Cooperativity and ion pairing in magnesium sulfate aqueous solutions from the dilute regime to the solubility limit†
Abstract
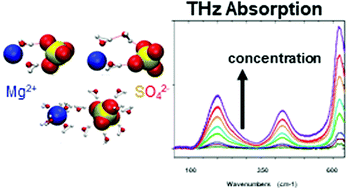
We report a terahertz absorption spectroscopy study of MgSO4 aqueous solutions in the concentration range 0.1 mol dm−3 to 2.4 mol dm−3. Accompanying classical MD simulations were carried out that use a polarizable force field parameterized to reproduce the solution thermodynamics. Contrary to prior reports, we find no evidence of contact ion pairs, even close to the solubility limit. Only solvent separated and different types of solvent shared ion pairs are found, being abundant even at the lowest concentration investigated here. The structure of the solution is concentration-dependent: the number of both types of ion pairs grows with increasing salt concentration. The combined theoretical and experimental analysis of the spectra in the frequency region 50–640 cm−1 suggests that the dynamics of water directly between two ions in solvent shared configuration is very strongly perturbed, via a cooperative, supra-additive, effect arising from the two ions. At high concentrations, the results support a scenario, where the perturbations in the water dynamics extend up to the third hydration layer via a cooperative, but additive, effect involving multiple ions. The SO42− and its hydration shell are much more strongly perturbed by the presence of the counterions than the first hydration shell of Mg2+. It is further shown that our simulations and observations are in agreement with thermodynamic properties of aqueous MgSO4 solutions derived by other methods.


 Please wait while we load your content...
Please wait while we load your content...