Symmetry and 1H NMR chemical shifts of short hydrogen bonds: impact of electronic and nuclear quantum effects†
Abstract
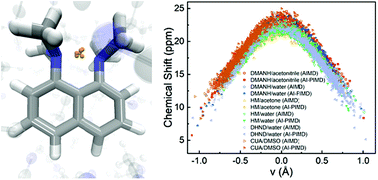
Short hydrogen bonds (SHBs), which have donor and acceptor separations below 2.7 Å, occur extensively in small molecules and proteins. Due to their compact structures, SHBs exhibit prominent covalent characters with elongated Donor–H bonds and highly downfield (>14 ppm) 1H NMR chemical shifts. In this work, we carry out first principles simulations on a set of model molecules to assess how quantum effects determine the symmetry and chemical shift of their SHBs. From simulations that incorporate the quantum mechanical nature of both the electrons and nuclei, we reveal a universal relation between the chemical shift and the position of the proton in a SHB, and unravel the origin of the observed downfield spectral signatures. We further develop a metric that allows one to accurately and efficiently determine the proton position directly from its 1H chemical shift, which will facilitate the experimental examination of SHBs in both small molecules and biological macromolecules.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...