Photoemission from non-polar aromatic molecules in the gas and liquid phase†
Abstract
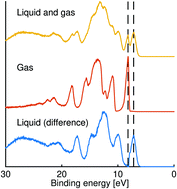
The photoelectron spectra of both liquid and gas phase aromatic molecules are reported. The spectra were obtained using a 34.1 eV source produced by high harmonic generation and analysed with the help of high-level ab initio simulations using the reflection principle combined with path integral molecular dynamics simulations accounting for nuclear quantum effects for the gas phase. We demonstrate the suitability of three trimethylbenzenes (1,3,5-trimethylbenzene, 1,2,3-trimethylbenzene and 1,2,4-trimethylbenzene) as a solvent for liquid photoelectron spectroscopy of solute species. We also discuss the electrokinetic charging of a non-polar liquid jet.


 Please wait while we load your content...
Please wait while we load your content...