Structural and electronic properties of NaTaO3 cubic nanowires†
Abstract
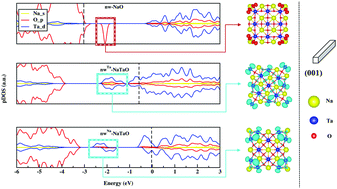
Sodium tantalate nanostructures have been classified among the best materials to conduct photocatalytic reactions. Therefore, understanding the relationship between nanoscale surface phenomena and photocatalytic properties is of fundamental importance. We performed Density Functional Theory calculations to investigate how chemically different facets may affect intrinsic properties of NaTaO3 cubic nanowires. Besides half-metallicity, the NaO-terminated wire relaxes structurally, presenting unoccupied down O 2p levels located above its valence band due to severely reduced coordination of its edges, which may allow it to be applied in spintronics systems. NaTaO-terminated wires have surface TaO4 units that, upon structural reconstruction, become more planar and introduce occupied Ta 5d levels below their conduction band. The emergence of such levels is also related to the overlap of Ta dz2 orbitals from adjacent NaTaO facets. Amongst other properties discussed herein, localized levels may be relevant for photocatalysis not only in terms of intrinsic bandgap engineering but also concerning the alignment with water redox potentials.


 Please wait while we load your content...
Please wait while we load your content...