Operando XANES from first-principles and its application to iridium oxide†
Abstract
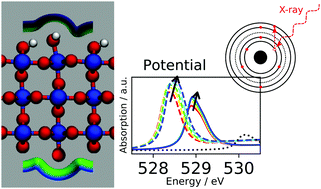
Efficient electro-catalytic water-splitting technologies require suitable catalysts for the oxygen evolution reaction (OER). The development of novel catalysts could benefit from the achievement of a complete understanding of the reaction mechanism on iridium oxide (IrO2), an active catalyst material that is, however, too scarce for large-scale applications. Considerable insight has already been provided by operando X-ray absorption near-edge structure (XANES) experiments, which paved the way towards an atomistic description of the catalyst's evolution in a working environment. We combine here first-principles simulations augmented with a continuum description of the solvent and electrolyte to investigate the electrochemical stability of various IrO2 interfaces and to predict the XANES cross-section for selected terminations under realistic conditions of applied potential. The comparison of computed O K-edge XANES spectra to corresponding experiments supports the formation of electron-deficient surface oxygen species in the OER-relevant voltage regime. Furthermore, surface hydroxyl groups that are found to be stable up to ∼1 V are suggested to be progressively oxidized at larger potentials, giving rise to a shift in the Ir L3-edge cross-section that qualitatively agrees with measurements.
- This article is part of the themed collection: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...