Cryo-temperature effects on membrane protein structure and dynamics†
Abstract
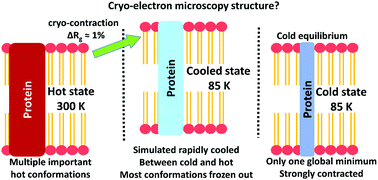
Innovations in cryogenic electron microscopy (Cryo-EM) have led to high-quality structures of important proteins such as the ribosome and γ-secretase, the membrane protease that produces Aβ involved in Alzheimer's disease. However, freezing may change protein structure and dynamics relative to the physiologically relevant “hot” state. To explore this, we studied substrate-bound γ-secretase (6IYC) by molecular dynamics as a hot, cold, and quickly cooled state in both membrane and water systems. We show that the experimental structure resembles the simulated cooled state, structurally between the hot and cold states and membrane and water systems, but with cold dynamics. We observe “cryo-contraction” in the membrane from 303 to 85 K, reducing radius of gyration (Rg) by 1% from 4.01 to 3.97 nm (6IYC = 3.95 nm). The hot state features an unwound C83-substrate with 10–14 α-helix residues (6IYC: 11) in equilibrium with an intact state with 16 helix residues not previously reported. The β-sheet is weakened with temperature. Multiple hot conformations probably control the Aβ42/Aβ40 ratio. We thus propose that MD simulation protocols of hot, cold, and cooled states as applied here can correct cryo-EM coordinates. However, important frozen-out fast modes require specific supplementary hot simulations or experiments.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...