Thermochemical studies of reactions of Re+ with SO2 using guided ion beam experiments and theory†
Abstract
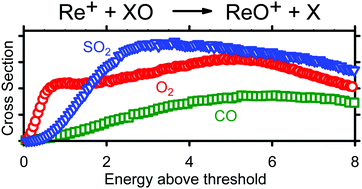
The kinetic energy dependent reactions of Re+ with SO2 were studied with guided ion beam tandem mass spectrometry. ReO+, ReO2+, and OReS+ species were observed as products, all in endothermic reactions. Modeling of the kinetic energy dependent cross sections yields 0 K bond dissociation energies (BDEs, in eV) of 4.78 ± 0.06 (Re+–O), 5.75 ± 0.02 (Re+–O2), and 4.35 ± 0.14 (Re+–SO). The latter two values can be combined with other information to derive the additional values 6.05 ± 0.05 (ORe+–O) and 4.89 ± 0.19 (ORe+–S). BDEs of ReO+ and ReO2+ agree with literature values whereas the values for OReS+ are the first measurements. The former result is obtained even though formation of ground state ReO+ is spin-forbidden. Quantum mechanical calculations at the B3LYP level of theory with a def2-TZVPPD basis set yield results that agree reasonably well with experimental values. Additional calculations at the BP86 and CCSD(T) levels of theory using def2-QZVPPD and aug-cc-pVxZ (x = T, Q, and 5) basis sets were performed to compare thermochemistry with experiment to determine that ReO2+ rather than the isobaric ReS+ is formed. Product ground states are 3Δ3(ReO+), 3B1(OReO+), 5Π−1(ReS+), and 3A′′(OReS+) after including empirical spin–orbit corrections, which means that formation of ground state products is spin-forbidden for all three product channels. The potential energy surfaces for the ReSO2+ system were also explored at the B3LYP/def2-TZVPPD level and exhibited no barriers in excess of the endothermicities for all products. BDEs for rhenium oxide and sulfide diatomics and triatomics are compared and discussed. The present result for formation of ReO+ is compared to that for formation of ReO+ in the reactions of Re+ + O2 and CO, where the former system exhibited interesting dual cross section features. Results are consistent with the hypothesis that the distinction of in-plane and out-of-plane CS symmetry in the triatomic systems might be the explanation for the two endothermic features observed in the Re+ + O2 reaction.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...