Perfluoroolefin complexes versus perfluorometallacycles and perfluorocarbene complexes in cyclopentadienylcobalt chemistry†
Abstract
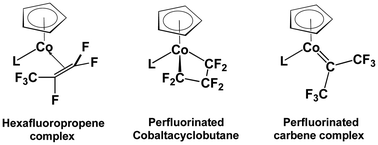
Fluorocarbons have been shown experimentally by Baker and coworkers to combine with the cyclopentadienylcobalt (CpCo) moiety to form fluoroolefin and fluorocarbene complexes as well as fluorinated cobaltacyclic rings. In this connection density functional theory (DFT) studies on the cyclopentadienylcobalt fluorocarbon complexes CpCo(L)(CnF2n) (L = CO, PMe3; n = 3 and 4) indicate structures with perfluoroolefin ligands to be the lowest energy structures followed by perfluorometallacycle structures and finally by structures with perfluorocarbene ligands. Thus, for the CpCo(L)(C3F6) (L = CO, PMe3) complexes, the perfluoropropene structure has the lowest energy, followed by the perfluorocobaltacyclobutane structure and the perfluoroisopropylidene structure less stable by 8 to 11 kcal mol−1, and the highest energy perfluoropropylidene structure less stable by more than 12 kcal mol−1. For the two metal carbene structures Cp(L)Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CF3)2 and Cp(L)Co
C(CF3)2 and Cp(L)Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF
CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.
CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.


 Please wait while we load your content...
Please wait while we load your content...