Reaction pathways and kinetics study on a syngas combustion system: CO + HO2 in an H2O environment†
Abstract
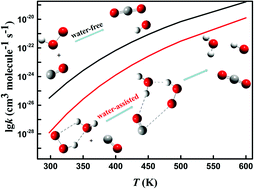
The reaction between CO and HO2 plays a significant role in syngas combustion. In this work, the catalytic effect of single-molecule water on this reaction is theoretically investigated at the CCSD(T)/aug-cc-pV(D,T,Q)Z and CCSD(T)-F12a/jun-cc-pVTZ levels in combination with the M062X/aug-cc-pVTZ level. Firstly, the potential energy surface (PES) of CO + HO2 (water-free) is revisited. The major products CO2 + OH are formed via a cis- or a trans-transition state (TS) channel and the formation of HCO + O2 is minor. In the presence of water, the title reaction has three different pre-reactive complexes (i.e., RC2: CO⋯HO2 + H2O, RC3: CO⋯H2O + HO2, and RC4: HO2⋯H2O + CO), depending on the initial hydrogen bond formation. Compared to the water-free process, the reaction barriers of the water-assisted process are reduced considerably, due to more stable cyclic TSs and complexes. The rate constants for the bimolecular reaction pathways CO + HO2, RC2, RC3, and RC4 are further calculated using conventional transition state theory (TST) with Eckart asymmetric tunneling correction. For reaction CO + HO2, our calculations are in good agreement with the literature. In addition, the effective rate constants for the water-assisted process decrease by 1–2 orders of magnitude compared to the water-free one at a temperature below 600 K. In particular, the effective rate constants for the water-assisted and water-free processes are 1.55 × 10−28 and 3.86 × 10−26 cm3 molecule−1 s−1 at 300 K, respectively. This implies that the contribution of a single molecule water-assisted process is small and cannot accelerate the title reaction.


 Please wait while we load your content...
Please wait while we load your content...