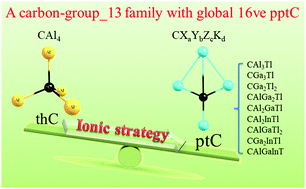
A sixteen-valence-electron carbon-group 13 family with global penta-atomic planar tetracoordinate carbon: an ionic strategy†
Abstract
The design of planar tetracoordinate carbon (ptC) has always been a challenge due to its unique bonding mode that necessitates the perfect balance between the carbon center and surrounding ligands both electronically and mechanically. A unique type of 18-valence-electron (18ve) template, i.e., CAl42−, has been found to be very effective in designing various novel 18ve-species upon skeletal substitution. In this work, we showed that though ptC is not the global structure for the parent 16ve-CAl4, suitable skeletal substitution can allow for a series of global minimum ptC species. Theoretical calculations at the level of CCSD(T)/def2-QZVP//B3LYP/def2-QZVP for 35 carbon-group 13 systems with 16-ve, i.e., CXaYbZcKd (X, Y, Z, K = Al/Ga/In/Tl; 0 ≤ a, b, c, d ≤ 4, a + b + c + d = 4), showed that 9 systems (CAl3Tl, CGa3Tl, CGa2Tl2, CAl2GaTl, CAl2InTl, CGa2InTl, CAlGa2Tl, CGa2InTl and CAlGaInTl) possess global minimum ptC and 2 systems (CAl3In and CAl2Tl2) have quasi-GM ptC. Except for CAl3Tl and CAl3In, all the ptCs were predicted for the first time. All these stable ptC structures have the same skeleton and can be described as the same ionic sub-structure, i.e., [A−]B+. This study not only enriches 16ve-ptC, but also directly demonstrates that utilizing an ionic strategy, non-ptC CAl4 also can be used as a template to extend the ptC family.


 Please wait while we load your content...
Please wait while we load your content...