A microfluidic study of synergic liquid–liquid extraction of rare earth elements†
Abstract
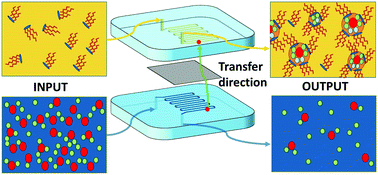
A microfluidic technique is coupled with X-ray fluorescence in order to investigate the origin of the so-called synergy effect observed in liquid–liquid extraction of rare earth elements (REEs) when special combinations of two extractants – one solvating and one ionic – are used. The setup enables kinetic studies by varying the two phases’ contact time. The results obtained are compared with those obtained using a standard batch extraction method at identical contact time. We then determine variations of free energies of transfer for five rare earth elements present in a solution together with a non-target ion (Fe3+) at different pH. Analysis of the effect of temperature and of surface charge density of the coexisting cations allows separating electrostatic effects from complexation effects. We finally show that all non-linear (synergic) effects are quadratic in mole fraction. This demonstrates that in-plane mixing entropy of the bent extractant film, in the first nanometer around rare earth ions, is the determining term in the synergy effect. Surprisingly, even when the third phase is present, free energies of transfer could still be measured in the dilute phase, which is reported for the first time, to our knowledge. We hence show that the extractive power of the dense third phase is stronger than that of conventional reverse aggregates in equilibrium with excess water.


 Please wait while we load your content...
Please wait while we load your content...