Vibrational dynamics in lead halide hybrid perovskites investigated by Raman spectroscopy†
Abstract
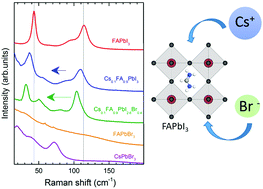
Lead halide perovskite semiconductors providing record efficiencies of solar cells have usually mixed compositions doped in A- and X-sites to enhance the phase stability. The cubic form of formamidinium (FA) lead iodide reveals excellent opto-electronic properties but transforms at room temperature (RT) into a hexagonal structure which does not effectively absorb visible light. This metastable form and the mechanism of its stabilization by Cs+ and Br− incorporation are poorly characterized and insufficiently understood. We report here the vibrational properties of cubic FAPbI3 investigated by DFT calculations on phonon frequencies and intensities, and micro-Raman spectroscopy. The effects of Cs+ and Br− partial substitution are discussed. We support our results with the study of FAPbBr3 which expands the identification of vibrational modes to the previously unpublished low frequency region (<500 cm−1). Our results show that the incorporation of Cs+ and Br− leads to the coupling of the displacement of the A-site components and weakens the bonds between FA+ and the PbX6 octahedra. We suggest that the enhancement of α-FAPbI3 stability can be a product of the release of tensile stresses in the Pb–X bond, which is reflected in a red-shift of the low frequency region of the Raman spectrum (<200 cm−1).


 Please wait while we load your content...
Please wait while we load your content...