Optimizing photon upconversion by decoupling excimer formation and triplet triplet annihilation†
Abstract
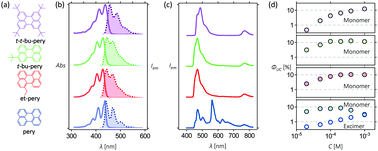
Perylene is a promising annihilator candidate for triplet–triplet annihilation photon upconversion, which has been successfully used in solar cells and in photocatalysis. Perylene can, however, form excimers, reducing the energy conversion efficiency and hindering further development of TTA-UC systems. Alkyl substitution of perylene can suppress excimer formation, but decelerate triplet energy transfer and triplet–triplet annihilation at the same time. Our results show that mono-substitution with small alkyl groups selectively blocks excimer formation without severly compromising the TTA-UC efficiency. The experimental results are complemented by DFT calculations, which demonstrate that excimer formation is suppressed by steric repulsion. The results demonstrate how the chemical structure can be modified to block unwanted intermolecular excited state relaxation pathways with minimal effect on the preferred ones.


 Please wait while we load your content...
Please wait while we load your content...