X-ray photoelectron and ion scattering spectroscopic surface analyses of amorphous and crystalline calcium phosphate nanoparticles with different chemical histories
Abstract
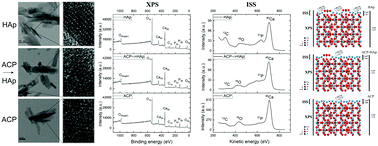
Abundant hydration, diffusivity, and volatile phase composition render the surface of calcium phosphates (CPs) a complex dynamic region. Three CP powders were analyzed using X-ray photoelectron spectroscopy (XPS) and ion scattering spectroscopy (ISS) with regard to their surface compositions and fine structures: hydroxyapatite (HAp), amorphous CP (ACP), and a CP formed under the same conditions as ACP but allowed to ripen into HAp (ACP → HAp). XPS analyses revealed that the Ca/P atomic ratio in the 2–10 nm-thick layers of the nanoparticle surface was somewhat lower than the stoichiometric ratio for all the three CPs. However, it was still lower for ACP and ACP → HAp than that for HAp, indicating the incongruent dissolution of all the surfaces and also greater instability and higher solubility of ACP and ACP → HAp as compared to those of HAp. Consequently, as indicated by both XPS and ISS, the amount of adventitious carbon bound to HAp was higher than that on ACP or ACP → HAp. The binding energies of the most intense XPS lines of all the three main atomic elements in HAp, i.e., O1s, P2p, and Ca2p downshifted for HAp as compared to those for ACP and ACP → HAp, supporting the premise of similarity in the surface structures between the two CP powders with a common amorphous precursor and kinetic path of formation. The ISS analysis, which investigated the 1–2 topmost atomic layers of the surface, indicated a higher level of heterogeneity of the oxygen states. This coincided with 40Ca accounting for over 80% atoms in this uppermost atomic layer of the surface of all three CPs. Ca–O bonds were particularly dominant in the topmost surface layer of ACP, where the Ca/P atomic ratio was an order of magnitude higher than that in ACP → HAp or HAp. A thermodynamic explanation and structural model of the surface accounting for the overabundance of Ca2+ in the topmost layer and the overall depletion of it elsewhere are provided in the discussion. Overall, the combined results of XPS and ISS analyses demonstrate a similarity between the surfaces of the two different forms of HAp as compared to that for ACP, but also a definite impression of the traces of their formation history on them.


 Please wait while we load your content...
Please wait while we load your content...