Cooperative effects of Na+ and citrates on the dissolution of calcium oxalate crystals†
Abstract
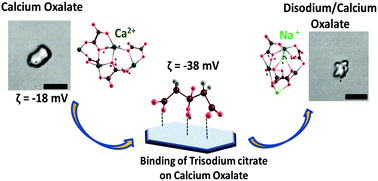
Precipitation and dissolution of calcium oxalate monohydrate (CaOx) crystals are relevant due to their major role in kidney stone diseases. To such an extent, small molecules and ions can act as inhibitors to prevent the formation of calcium oxalate monohydrate crystals. Herein, we explored the role of citrate and the counter cation Na+ ions in the dissolution of CaOx crystals. Citrate binds on the Ca2+ sites of the CaOx crystals to form calcium citrate. Dissolution of CaOx increases with the increase in the concentration of citrate ions and time of incubation. We observed that corrugations were formed on the surface of the CaOx crystals after the sodium citrate treatment during the dissolution process. Theoretical studies revealed that Na+ occupies the vacant site of Ca2+ in CaOx making a strain on the surface which leads to the subsequent deformation of the crystal.


 Please wait while we load your content...
Please wait while we load your content...