Quantum kinetic energy and isotope fractionation in aqueous ionic solutions
Abstract
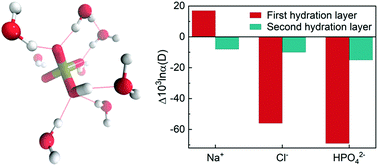
At room temperature, the quantum contribution to the kinetic energy of a water molecule exceeds the classical contribution by an order of magnitude. The quantum kinetic energy (QKE) of a water molecule is modulated by its local chemical environment and leads to uneven partitioning of isotopes between different phases in thermal equilibrium, which would not occur if the nuclei behaved classically. In this work, we use ab initio path integral simulations to show that QKEs of the water molecules and the equilibrium isotope fractionation ratios of the oxygen and hydrogen isotopes are sensitive probes of the hydrogen bonding structures in aqueous ionic solutions. In particular, we demonstrate how the QKE of water molecules in path integral simulations can be decomposed into translational, rotational and vibrational degrees of freedom, and use them to determine the impact of solvation on different molecular motions. By analyzing the QKEs and isotope fractionation ratios, we show how the addition of the Na+, Cl− and HPO42− ions perturbs the competition between quantum effects in liquid water and impacts their local solvation structures.
- This article is part of the themed collections: 2020 PCCP HOT Articles and Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...