Controlling “like–likes–like” charge attraction in hydroxy-functionalized ionic liquids by polarizability of the cations, interaction strength of the anions and varying alkyl chain length
Abstract
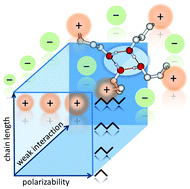
We provide comprehensive understanding of “like–likes–like” charge attraction in hydroxy-functionalized ionic liquids (ILs) by means of infrared spectroscopy (IR), quantum chemistry and differential scanning calorimetry (DSC). We show that hydrogen bonding between cation and cation (c–c) is possible despite the repulsive forces between ions of like charge. Already at room temperature, the (c–c) hydrogen bonds can compete with the regular Coulomb-enhanced hydrogen bonds between cation and anion (c–a). For a large set of well-selected ILs, we show that “like-charge attraction” between the OH-functionalized cations is controllable by the polarizability of the cation, the interaction strength of the anion and the length of the hydroxyalkyl chain. In particular, we clarify whether tethering the OH group away from the positive charge center of the cationic ring with longer hydroxyalkyl chains compensates for unfavourable cation/anion combinations with respect to (c–c) cluster formation. For that purpose, we synthesized and characterized twelve ionic liquids including the differently polarizable cations, 1-(n-hydroxyalkyl)-1-methylpiperidinium [HOCnMPip]+ and 1-(n-hydroxyalkyl)-pyridinium [HOCnPy]+, as well as the weakly and strongly interacting anions, bis(trifluoromethanesulfonyl)imide [NTf2]− and methanesulfonate [OMs]−, respectively. On top, we varied the hydroxyalkyl chain length (HOCn) (n = 2–5). We systematically show how these three molecular ion parameters affect like-charge attraction. The use of polarizable cations, weakly interacting anions, and long alkyl chain tethers results in (c–c) clustering already at room temperature. Kinetic trapping is not a prerequisite for the existence of (c–c) cluster species in ILs. Moreover, we demonstrate that micro structuring affects macroscopic behavior of this type of ILs. We observed that substantial (c–c) interaction prevents ILs from crystallizing. Instead, these ILs supercool and finally form a glass.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...