Branching effect on the linear and nonlinear optical properties of styrylpyrimidines†
Abstract
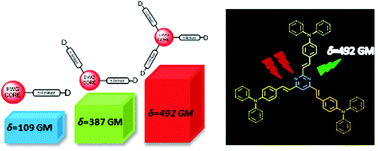
This contribution aims at investigating the branching effect on the steady state, time resolved fluorescence and two-photon absorption (2PA) properties of dimethylamino and diphenylamino substituted styrylpyrimidine derivatives, by means of a combined experimental and theoretical study. In contrast to classical branched molecules with a triphenylamine central core and electron accepting groups at the periphery, here, branched molecules with reverse topology and different symmetries are examined, namely a styrylpyrimidine group is used as the electron withdrawing core and dimethylamino or diphenylamino donors are incorporated at the periphery. Besides, compared to the great majority of existing branched systems, the herein studied molecules do not have C3 symmetry. For this reason, the region of the linear and non-linear optical spectra of the two and three branched chromophores is actually similar. Interestingly, while the one-photon absorption spectra of one-branched systems versus two- or three-branched ones are spectrally shifted, there is almost no spectral shift in the main 2PA spectral region. Meanwhile, there is still an enhancement of both linear and nonlinear optical responses. Overall, here we developed a strategy that enhances the 2PA response while maintaining the spectral position. Specifically, 2PA cross section values as high as 500 GM have been obtained for the diphenylamino A–(π–D)3 molecule in dichloromethane.


 Please wait while we load your content...
Please wait while we load your content...