Enhancing the stability of perovskites by constructing heterojunctions of graphene/MASnI3†
Abstract
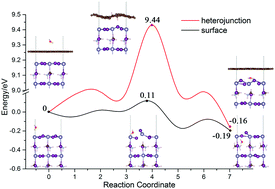
In this paper, the geometric and electronic properties of heterojunctions constructed using a graphene sheet and an MASnI3 surface were investigated by performing first-principles calculations based on the density functional theory. Our results show that the interaction between graphene and the MASnI3 surface is in the scope of van der Waals interactions. In the heterojunctions, electrons transfer from graphene to the MASnI3 surface, resulting in the formation of a built-in electric field in the interface, which is favorable for the separation of electrons and holes. The absorption spectra showed that the absorption intensity of the heterojunction in the visible region is slightly smaller than that of the pristine MASnI3 surface. The energy barriers of water molecules diffusing through MASnI3 surfaces are relatively low, but when a water molecule penetrates the graphene sheet into the interior of the MASnI3 it has to overcome an energy barrier of as high as 9 eV. It is found that the water diffusions through the surfaces cause very severe damage to the structures of the graphene sheet and MASnI3 surface. So, the graphene can block the penetration of water into the inside of the material and retard the degradation of the perovskites. Coating a graphene sheet onto the MASnI3 surface to form a heterojunction is an effective strategy of enhancing the stability and performance of perovskite solar cells. This study could provide an in-depth understanding of the properties of graphene/MASnI3 heterojunctions and contribute to the design strategy of perovskite-based solar cells.


 Please wait while we load your content...
Please wait while we load your content...